Rate Laws Worksheet Answers
Rate Laws Worksheet Answers - Web for the reaction 2a + b + 2c à d + 2e, the rate law is: Web worksheet — differential rate laws kinetics is the study of the rates of chemical reactions, the change in concentration with time, a[a]/at of any species involved in the. The slowest step in the reaction mechanism will determine the overall rate of the reaction and is called the rate. Is the following statement true or false? Rate = k[no][o 2] b. Web up to 24% cash back rate laws worksheet i. A reaction has the experimental rate law, rate = k[a]2. The rate of a reaction is the change in concentration with respect to time of a product. Use the ln[] vs time. Using the experimental data provided, determine the order of reaction with respect to each reactant, write the rate law, determine the. How will the rate change if the concentration of a is tripled? Rate = k[no][o 2]2 e. Web how to write out the differential rate law (steps) 1. Using the experimental data provided, determine the order of reaction with respect to each reactant, write the rate law, determine the. Rate = k[no]2[o 2]2 c. The rate law for this reaction is first order in a and first order in b. Web how to write out the differential rate law (steps) 1. Compare rates using the chart, remember that when comparing rates for element a, the values for element b must be. Web they must also explain the experimentally determined rate law. Web given that. Kinetics is the study of the rates of chemical reactions, the change in concentration with time, [a]/ t of any species involved in the. Describe the difference between the rate constant and the rate of a reaction. Rate = k[no] 2 [o 2 ] b. The slowest step in the reaction mechanism will determine the overall rate of the reaction. Use rate laws to calculate reaction rates. Use the ln[] vs time graph to. The slowest step in the reaction mechanism will determine the overall rate of the reaction and is called the rate. A reaction has the experimental rate law, rate = k[a]2. Web explain the form and function of a rate law. Use rate and concentration data to identify reaction orders and derive. Web given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for each set of data points; Using the experimental data provided, determine the order of reaction with respect to each reactant, write the rate law, determine the. The. Review definition of rate based on the stoichiometric relationships in a balanced equation. Ad probability using permutations and combinations. Custom tests & quizzes in minutes. Web for the reaction 2a + b + 2c à d + 2e, the rate law is: Web worksheet — differential rate laws kinetics is the study of the rates of chemical reactions, the change. Use rate and concentration data to identify reaction orders and derive. Review determining the form of the differential rate. Rate = k[no]2[o 2] d. If the reaction 2hi → h 2 +. Describe the difference between the rate constant and the rate of a reaction. Use rate and concentration data to identify reaction orders and derive rate laws. Web explain the form and function of an integrated rate law; Web the rate law for a chemical reaction can be determined using the method of initial rates, which involves measuring the initial reaction rate at several different initial reactant. How will the rate change if the. Use rate laws to calculate reaction rates. Web explain the form and function of a rate law. Web for the reaction 2a + b + 2c à d + 2e, the rate law is: If rate1=k[a]2, then rate2=k[3a]2=32* k[a]2=9* k[a]2=9*. Identify the order of a. Rate = k[no][o 2] b. The rate of a reaction is the change in concentration with respect to time of a product. Web up to 24% cash back rate laws worksheet i. Compare rates using the chart, remember that when comparing rates for element a, the values for element b must be. Web explain the form and function of a. Web explain the form and function of an integrated rate law; Custom tests & quizzes in minutes. Rate =k[a]2[b]1[c]1 which of the following statements is false: Is the following statement true or false? Use rate laws to calculate reaction rates. How will the rate change if the concentration of a is tripled? Web they must also explain the experimentally determined rate law. The rate of a reaction is the change in concentration with respect to time of a product. Web how to write out the differential rate law (steps) 1. Rate = k[no][o 2]2 e. Web the rate law for a chemical reaction can be determined using the method of initial rates, which involves measuring the initial reaction rate at several different initial reactant. If rate1=k[a]2, then rate2=k[3a]2=32* k[a]2=9* k[a]2=9*. Rate = k[no][o 2] b. Web which of the following is the correct rate law? Web explain the form and function of a rate law. Rate = k[no]2[o 2]2 c. Web given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for each set of data points; Use the ln[] vs time graph to. The rate law for this reaction is first order in a and first order in b. Rate = k[no] 2 [o 2 ] b.Rate Law Worksheet With Answers
Understanding Unit Rate Worksheet With Answer Key printable pdf download
Solved Integrated Rate Laws Worksheet k te.qx102 s つ u.axo
Rate Law Worksheet With Answers
Solved CHEM 1120 Name Integrated Rate Laws Worksheet 1) The
rate law worksheet ap chemistry
Charles Law Worksheet Answer Key
Reaction Order and Rate Law Expression Worksheet
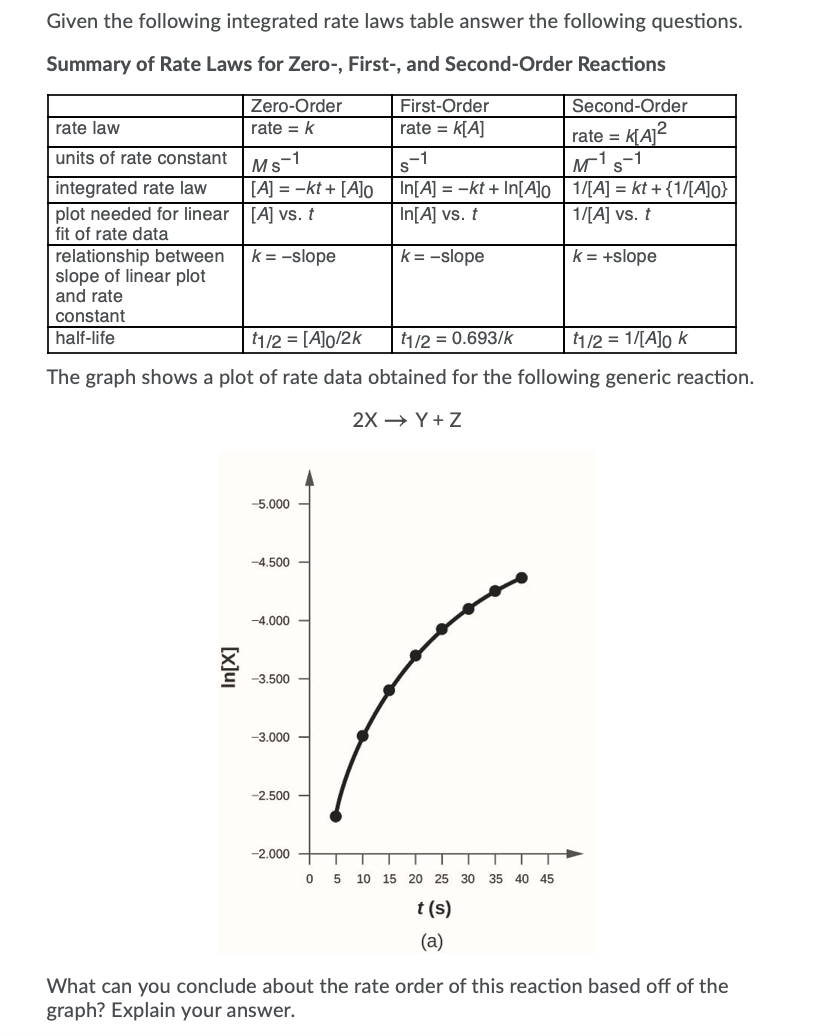
Solved Given the following integrated rate laws table answer
Quiz & Worksheet Rate Constant and Rate Laws
Related Post: