Chemical Equations Worksheet Answer Key
Chemical Equations Worksheet Answer Key - (coefficients equal to one (1) do not need to be shown in your answers). Write a balanced chemical equation for each of the word equations below. 3f 2 + 2fei 3 → 3i 2 + 2fef 3 ; Web answer a \(\ce{pcl5}(s)+\ce{h2o}(l)\rightarrow \ce{pocl3}(l)+\ce{2hcl}(aq)\) answer b \(\ce{3cu}(s)+\ce{8hno3}(aq)\rightarrow. 4 fe+ 3 o2 − − → 2 fe 2o3. Identify the reactants and the products. ____ h2 + ____ o2 ____ h2o. Zn + fe(no 3) 2 → zn(no 3) 2 + fe; 2 fe+ 3 cl2 − − → 2 fecl3. Place the products on the right. N2 + 3 h2 æ 2 nh3. Balancing more complex chemical equations. Vocabulary:avogadro窶冱 number, chemical equation, chemical formula, chemical reaction,coefficient, combination, combustion,. 2 nacl + 1 f2 æ 2 naf + 1 cl2. The law of conservation of matter states that, in a system that is closed to the transfer of matter, the amount of matter in the. 2 nacl + 1 f2 æ 2 naf + 1 cl2. Ad unravel the mysteries of the world with informative books on various topics. ____ nacl + ____ f2 ____ naf + ____ cl2. One element replaces another element in a compound. Web balance the equations below: Sn + h 2 so 4 → snso 4 + h 2; Often, students lose hope and struggle to solve it. Balance the overall equation using coefficients. In each case, a molecule consists of two or more combined. Identify the reactants and the products. 2 fe+ 3 cl2 − − → 2 fecl3. 3f 2 + 2fei 3 → 3i 2 + 2fef 3 ; Balance the overall equation using coefficients. Web the resources above cover the following skills: If you are struggling as well, then all you need balancing equations worksheet with answers. Web heating and cooling curves answer key; Software for math teachers that creates custom worksheets in a matter of minutes Table of contents [ show] understanding the methods and tips can make it easier for you to balance the chemical equation. Kclo3 æ 2 kcl + 3 o2. 2 nacl + 1 f2 æ 2 naf + 1 cl2. 2 nacl + 1 f2 æ 2 naf + 1 cl2. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2. Place the reactants on the left. 2 h2 + 1 o2 æ 2.. Heating and cooling curves part 2. 2 h2 + 1 o2 æ 2. 2 fe+ 3 cl2 − − → 2 fecl3. N2 + 3 h2 æ 2 nh3. Web steps for writing chemical equations. ____ h2 + ____ o2 ____ h2o. 2 fe+ 3 cl2 − − → 2 fecl3. N2 + 3 h2 æ 2 nh3. ____ nacl + ____ f2 ____ naf + ____ cl2. Aqueous sodium chloride reacts with. 4 fe+ 3 o2 − − → 2 fe 2o3. Software for math teachers that creates custom worksheets in a matter of minutes 2 h2 + 1 o2 æ 2. Vocabulary:avogadro窶冱 number, chemical equation, chemical formula, chemical reaction,coefficient, combination, combustion,. ____ n2 + ____ h2 ____ nh3. 2 fe+ 3 cl2 − − → 2 fecl3. Web steps for writing chemical equations. ____ h2 + ____ o2 ____ h2o. ____ n2 + ____ h2 ____ nh3. Aqueous sodium chloride reacts with. Zn + fe(no 3) 2 → zn(no 3) 2 + fe; ____ h2 + ____ o2 ____ h2o. Balance the overall equation using coefficients. Web answer a \(\ce{pcl5}(s)+\ce{h2o}(l)\rightarrow \ce{pocl3}(l)+\ce{2hcl}(aq)\) answer b \(\ce{3cu}(s)+\ce{8hno3}(aq)\rightarrow. N2 + 3 h2 æ 2 nh3. Visually understanding balancing chemical equations. 2 fe+ 3 cl2 − − → 2 fecl3. ____ kclo3 ____ kcl + ____ o2. Place the products on the right. Heating and cooling curves part 2 answer key; N2 + 3 h2 æ 2 nh3. Often, students lose hope and struggle to solve it. Aqueous sodium chloride reacts with. Web every balanced chemical equation consists of two parts: Kclo3 æ 2 kcl + 3 o2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms,. Place the reactants on the left. 2 h2 + 1 o2 æ 2. Sn + h 2 so 4 → snso 4 + h 2; 2 nacl + 1 f2 æ 2 naf + 1 cl2.Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf
Balancing Chemical Equations Worksheet Answer Key 110 › Athens Mutual
Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf
Balancing Chemical Equations Worksheet Grade 10
Balancing Chemical Equations Worksheets With Answers Business Mentor
Chemistry Balancing Chemical Equations Worksheet Answer Key —
49 Balancing Chemical Equations Worksheets [with Answers]
Unit 7 Balancing Chemical Equations Worksheet 2 Answer Key Tessshebaylo
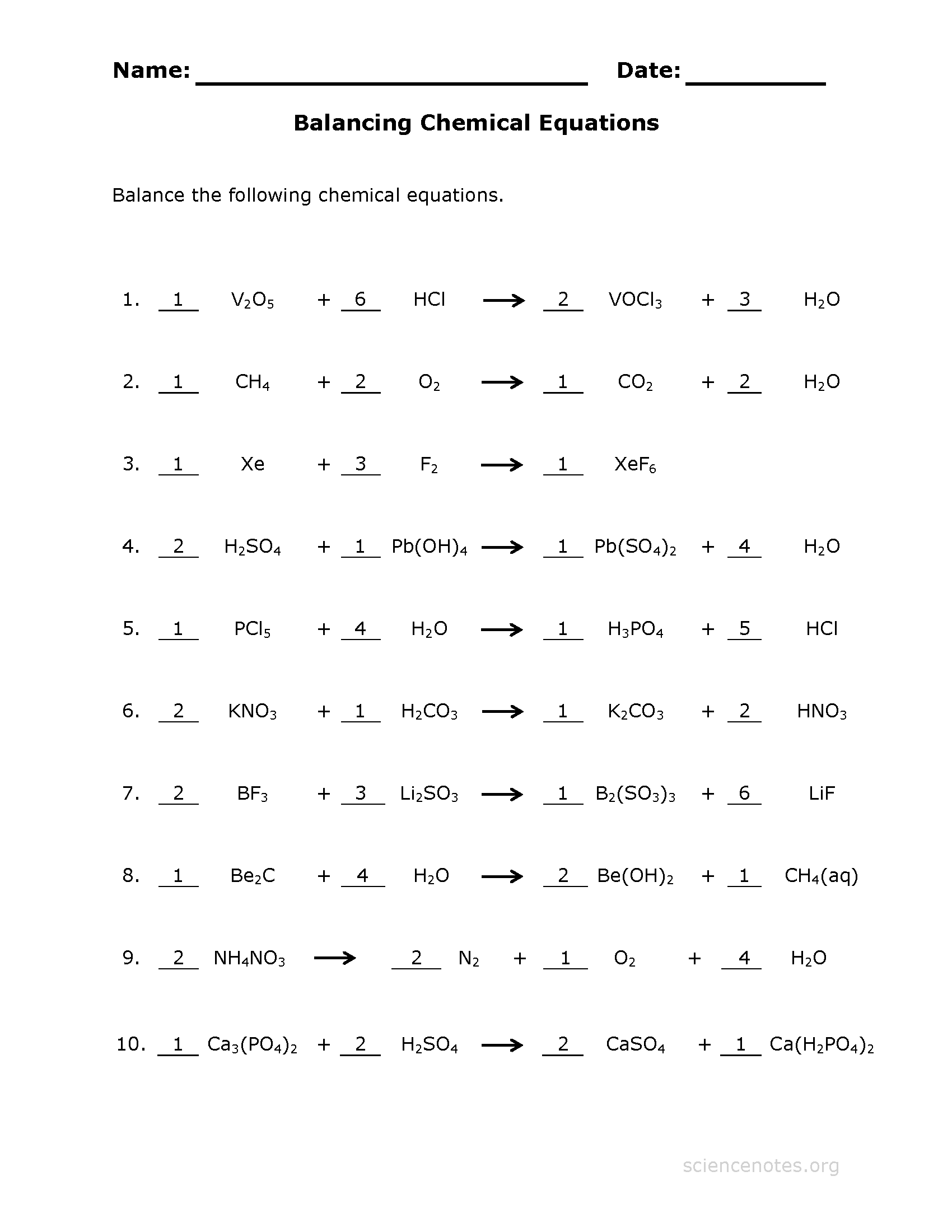
Balance Chemical Equations Worksheet 3 Answer Key Science Notes and
Balancing Chemical Equations Worksheets 1 Answer Key
Related Post:






![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-02.jpg)