Calculating Percent Abundance Of Isotopes Worksheet
Calculating Percent Abundance Of Isotopes Worksheet - Distinguish between atomic weight, atomic. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Web worksheet to help students calculate ram from relative abundances of isotopes. Isotopes and average atomic mass is shared under a not declared license and was authored, remixed, and/or curated by libretexts. The average atomic gemessene on the. Explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. Web the percent abundance of these isotopes is as follows: A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. Web this problem demonstrates finding the percent abundance of isotopes with known average atomic mass. Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. The average atomic mass of the three isotopes is 24.3050 amu. Calculate the atomic mass of boron. Web 🎯 want to ace chemistry? Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b (11.009 amu*). Let’s now suppose we need to determine the percent abundance of the two main isotopes of copper: 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Isotopes and average atomic mass is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Structured to start by thinking of balls in a box, through to simple calculation. Web this problem demonstrates finding the percent abundance of isotopes with known average atomic mass. Web the percent abundance. Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written as a decimal. 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99 amu, and 8.82% abundance with 21.99. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring. Distinguish between atomic weight, atomic. Web percent abundance of isotopes. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b (11.009 amu*). Web calculate atomic weight from percent abundance ; A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. Isotopes and average atomic mass is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Manipulate the atomic weight equation to calculate various unknown variables ; A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. One isotope has a mass of 63.00 amu and. Web the natural abundance for. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Calculate the atomic mass of boron. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. Web worksheet to help students calculate ram from relative abundances of isotopes. 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99. Distinguish between atomic weight, atomic. Web calculate atomic weight from percent abundance ; Isotopes and average atomic mass is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written as. 65 cu and 63 cu. Web 🎯 want to ace chemistry? Structured to start by thinking of balls in a box, through to simple calculation. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. The average atomic gemessene on the. Web percent abundance of isotopes. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written as a decimal. Web 🎯 want to ace chemistry? Isotopes and average. 65 cu and 63 cu. Using the atomic mass on the periodic table for an element with two isotopes,. Distinguish between atomic weight, atomic. Web 🎯 want to ace chemistry? Manipulate the atomic weight equation to calculate various unknown variables ; Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. Let’s now suppose we need to determine the percent abundance of the two main isotopes of copper: 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99 amu, and 8.82% abundance with 21.99. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. Manipulate the atomic weight equation to calculate various unknown variables ; One isotope has a mass of 63.00 amu and. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. Using the atomic mass on the periodic table for an element with two isotopes,. The average atomic mass of the three isotopes is 24.3050 amu. Web worksheet to help students calculate ram from relative abundances of isotopes. Isotopes of an element are atoms with. 65 cu and 63 cu. Web this problem demonstrates finding the percent abundance of isotopes with known average atomic mass. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. The average atomic gemessene on the. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b (11.009 amu*). Web an element has the following natural abundances and isotopic masses:How To Calculate Relative Abundances Of Two Isotopes
Isotopes and Relative Atomic Mass GCSE Lesson (SC3c CC3c) Teaching
ShowMe percent abundance
How to Solve for Percent Abundance of Isotopes Examples, Practice
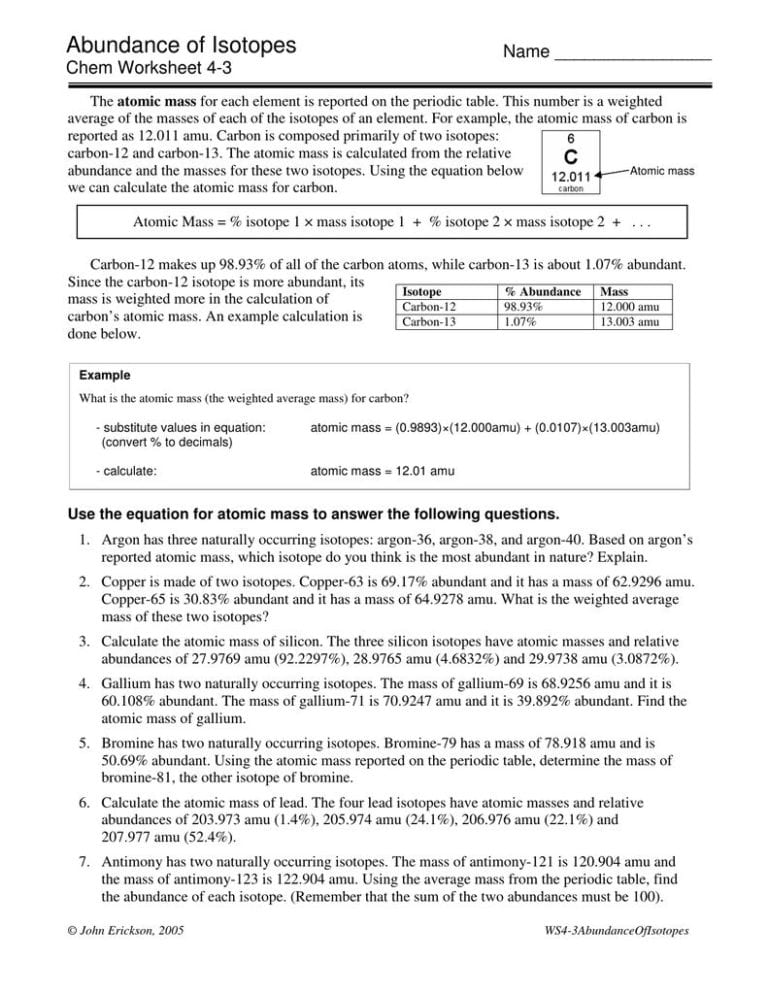
Abundance Of Isotopes Chem Worksheet 4 3 —
How To Find Relative Abundance Of 3 Isotopes
Abundance Of Isotopes Chem Worksheet 4 3 —
How To Calculate Percent Abundance Of Each Isotope
Average Atomic Mass and Percent Abundance Worksheet 2 and KEY Isotope
Abundance Of Isotopes Chem Worksheet 43
Related Post: