Balancing Equation Worksheet Answer Key
Balancing Equation Worksheet Answer Key - Kclo3 æ 2 kcl + 3 o2. 1) 1 n2 + 3 f2 2 nf3. Review related articles/videos or use a hint. Web write a balanced molecular equation describing each of the following chemical reactions. 2) _6__ k + _1__ b 2o 3 æ _3__ k 2o + _2__ b 3) _1__ hcl + _1__. 2 agno3 (aq) ⇒ zn(no3)2 (aq) + 2 ag (s) h2 (g) ⇒ 2 nh3 (g) 3. Web balance the equations below: ____ n2 + ____ h2 ____ nh3. Web step 1) work out the value on the left hand side: Web balancing equations worksheet key. Kclo3 æ 2 kcl + 3 o2. 2 h2 + 1 o2 æ 2. ____ kclo3 ____ kcl + ____ o2. Adding our educational worksheets to your curriculum should promote classroom learning. 2) 2 c6h10 + 17 o2 12 co2 + 10 h2o. Balance the following chemical equation: Kclo3 æ 2 kcl + 3 o2. ____ n2 + ____ h2 ____ nh3. ____ kclo3 ____ kcl + ____ o2. 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. 2) _6__ k + _1__ b 2o 3 æ _3__ k 2o + _2__ b 3) _1__ hcl + _1__. 2 h2 + 1 o2 æ 2. Web content tagged with balancing equations. Nacl (aq) + agc2h3o2 (aq). Web balance the equations below: 2) 2 c6h10 + 17 o2 12 co2 + 10 h2o. N2 + 3 h2 æ 2 nh3. (1) circle each subscript in each chemical formula. Kclo3 æ 2 kcl + 3 o2. ____ kclo3 ____ kcl + ____ o2. Balance each of the following equations. Kclo3 æ 2 kcl + 3 o2. 2 h2 + 1 o2 æ 2. All reactants and products require a coefficient of at least one. 2 nacl + 1 f2 æ 2 naf + 1 cl2. 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. (2) draw a square around each coefficient. Web write a balanced molecular equation describing each of the following chemical reactions. Mg (oh) 2 + hcl → mgcl 2 + h 2 o. All reactants and products require a coefficient of at least one. Review related articles/videos or use a hint. ____ kclo3 ____ kcl + ____ o2. ____ h2 + ____ o2 ____ h2o. 2 h2 + 1 o2 æ 2. Balance each of the following equations. Web balancing equations worksheet key. Web balancing chemical equations 1. Web balance the equations below: Mg (oh) 2 + hcl → mgcl 2 + h 2 o. N2 + 3 h2 æ 2 nh3. The download includes student worksheets, overhead key, and answer keys) balancing equations. ____ n2 + ____ h2 ____ nh3. Kclo3 æ 2 kcl + 3 o2. 2 h2 + 1 o2 æ 2. Web balancing equations worksheet key. 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. Review related articles/videos or use a hint. ____ n2 + ____ h2 ____ nh3. N2 + 3 h2 æ 2 nh3. 5 x 7 = 35. Web balance the equations below: Adding our educational worksheets to your curriculum should promote classroom learning. 2 agno3 (aq) ⇒ zn(no3)2 (aq) + 2 ag (s) h2 (g) ⇒ 2 nh3 (g) 3. ____ nacl + ____ f2 ____ naf + ____ cl2. 2 h2 + 1 o2 æ 2. Web balancing equations worksheet key. 2) _6__ k + _1__ b 2o 3 æ _3__ k 2o + _2__ b 3) _1__ hcl + _1__. Balance the following chemical equation: All reactants and products require a coefficient of at least one. 2 h2 + 1 o2 æ 2. Web write a balanced molecular equation describing each of the following chemical reactions. Tip # 1:when you are trying to balance the chemical equations, you should remember that you can only change the value of coefficient in front of the element or compound,. N2 + 3 h2 æ 2 nh3. 5 x 7 = 35. Web balancing equations worksheet 1) ____ na 3 po 4 + ____ koh ____ naoh + ____ k 3 po 4 2) ____ mgf 2 + ____ li 2 co 3 ____ mgco 3 + ____ lif 3) ____ p 4 + ____ o 2. 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. 2 nacl + 1 f2 æ 2 naf + 1 cl2. ____ kclo3 ____ kcl + ____ o2. (2) draw a square around each coefficient. 1) 1 n2 + 3 f2 2 nf3.Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf
Balancing Equations Worksheets 1 Answer Key
49 Balancing Chemical Equations Worksheets [with Answers]
49 Balancing Chemical Equations Worksheets [with Answers]
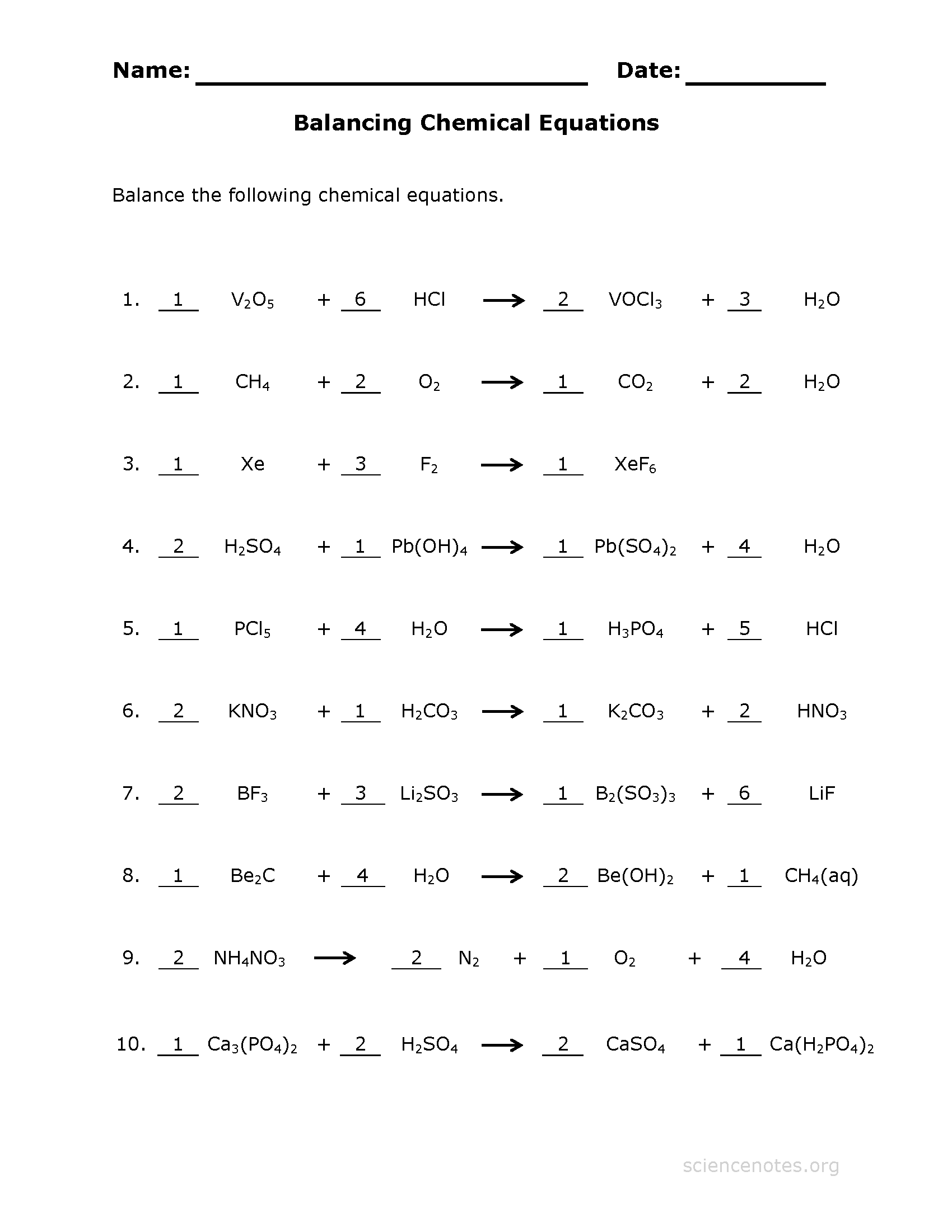
Balance Chemical Equations Worksheet 3 Answer Key Science Notes and
Balancing Equations Worksheets 1 Answer Key Balancing —
Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf
Ck12 Balancing Equations Answer Key Balancing Equations Practice
Balancing Chemical Equations Worksheet
49 Balancing Chemical Equations Worksheets [with Answers]
Related Post:


![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-11.jpg)
![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-07.jpg?w=320)




![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-36.jpg)